Beta Glucan: The Immune Boosting Wonder You’ve Been Searching For (Part 2/3)
Beta Glucans: What are they really?
Mushroom extracts are all the craze today. Have you ever wondered what are in these extracts? What do you as a consumer look out for when you buy a product? The answer to these questions is not very simple as there are an array of different bioactive molecules which give mushrooms their functional benefits. The three main classes of biomolecules being- polysaccharides, terpenes and phenolics. In this blog we will delve deeper into the world of polysaccharides, an important class of compounds that are responsible for giving mushrooms a lot of their healing properties. However, please do keep in mind that all polysaccharides are not the same, as we will see later in this blog. Our primary focus here will be Beta glucans, which are the main functional components of mushroom polysaccharides.
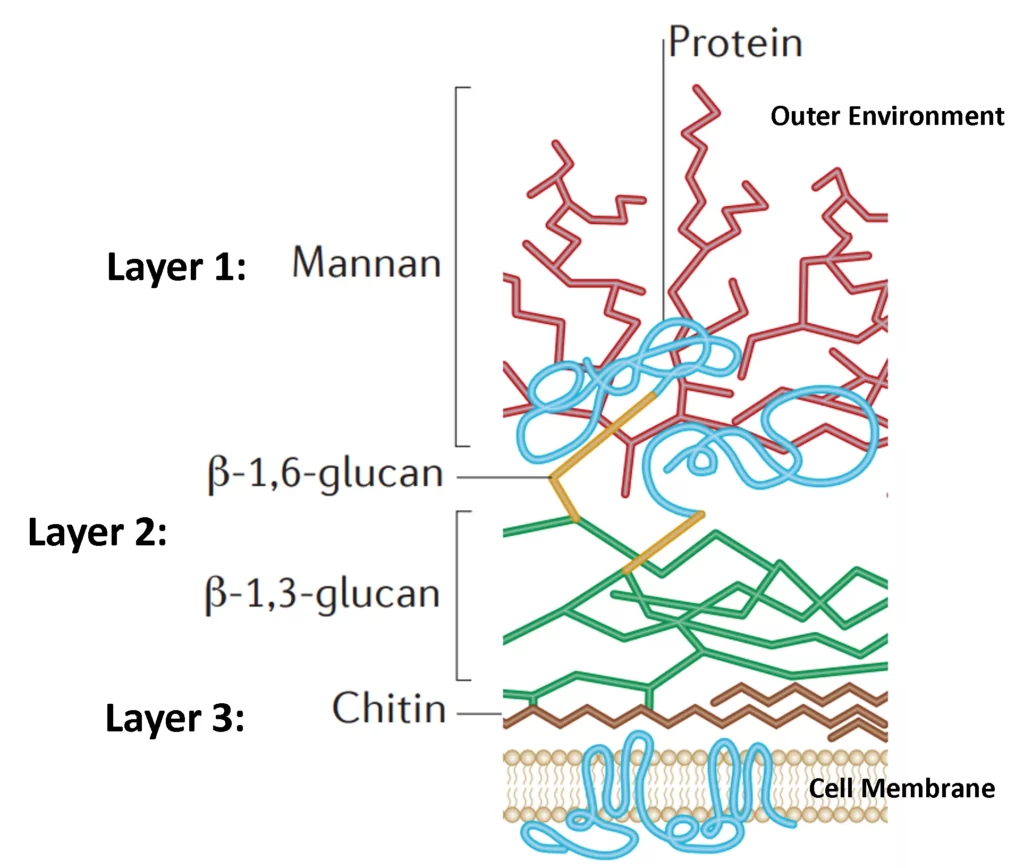
In our previous blog we had a look at the intricacies of the fungal cell wall. The three major layers (Chitin, beta glucans and mannoproteins) are intertwined into a dynamic barrier that has stood the tests of time and allowed fungi to thrive (See Figure 1). Having evolved with fungi for billions of years, our immune systems recognize these cell wall components as a threat and spring into action. These molecules are what give mushroom extracts their immuno-modulatory function. A major active ingredient in these extracts are the beta glucans. They are polysaccharides that form the middle layer of the fungal cell wall (Layer 2 in Fig 1).
Fig 1: Cartoon representation of a Candida albicans (yeast) cell wall (Source)
Beta glucans are incredibly diverse in their composition and function. Different mushrooms contain different beta glucan molecules. To fully understand these differences, we will be exploring these polysaccharides from a molecular perspective.
In this blog we will deconstruct the polysaccharide molecule and understand how they are named. We will also explore some of the common beta glucans obtained from mushrooms and their health benefits. By the end of this blog we hope that you will have a much better understanding of what beta glucans are and what to look for in your own research.
Before we delve into the details of beta glucans, we must first understand the term polysaccharide.
What are Polysaccharides
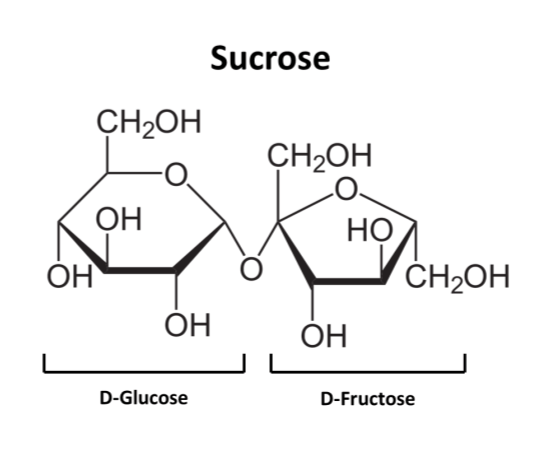
Any molecule composed of long chains of carbohydrates strung together is known as a polysaccharide (“poly” = ‘Many’, “Saccharide” = ‘Sugar’). These chains are made of sugars called monosaccharides. The most common examples of monosaccharides are glucose, mannose, fructose, galactose and xylose. Our beloved table sugar is in fact made up of glucose and fructose. Each molecule of sugar (sucrose) consists of one glucose and one fructose molecule. Since there are two monosaccharides in sucrose, it is known as a disaccharide. When the number of monosaccharides exceeds twelve the molecule is known as a polysaccharide.
Fig 2: Sucrose, a disaccharide made of D-Glucose and D-Fructose
Glucose: The building blocks of beta glucans
To understand how polysaccharides are named, we must first look at the molecular structure of a polysaccharide molecule. We start with the humble glucose molecule. Glucose is nature’s preferred fuel source. Almost all living things break down glucose within their cells to generate ATP (adenosine triphosphate) which is the energy source for biochemical reactions.
Structure of a Glucose Molecule
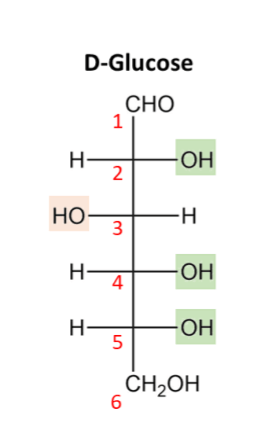
Glucose consists of 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms giving it the molecular formula of C6H12O6. The carbon atoms are arranged in a 6-member chain with the oxygen and hydrogen atoms sticking out from the central chain. Every carbon atom is attached to at least one hydrogen atom and either an alcohol (-OH) or ketone (=O) functional group. The distribution of these functional groups determines the name of the monosaccharide. Glucose is specifically the 6- carbon monosaccharide which has an arrangement of atoms as depicted in the figure below.
Fig 3: Structure of a D-glucose molecule in the open-chain format
Many Forms of Glucose
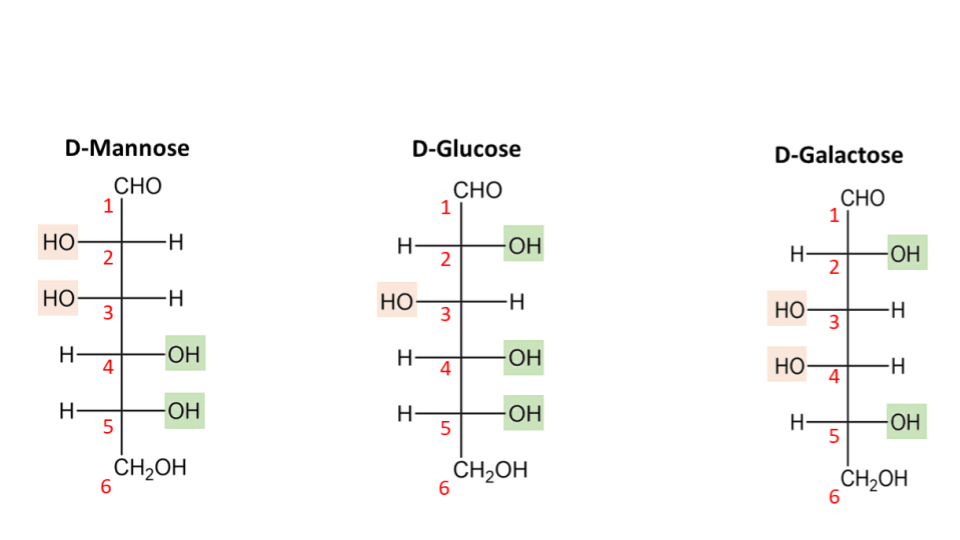
If one were to change the orientation of the -OH and -H molecules on any of the carbon atoms, we would obtain a different monosaccharide. For example, galactose and mannose have the same number of carbon, oxygen and hydrogen molecules as glucose but with different arrangements. Such molecules are known as isomers. Hence, mannose and galactose are isomers of glucose.
Fig 4: Isomers of D-glucose depicted in the open-chain format highlighting the distribution of functional groups across each molecule
The Ring of Power
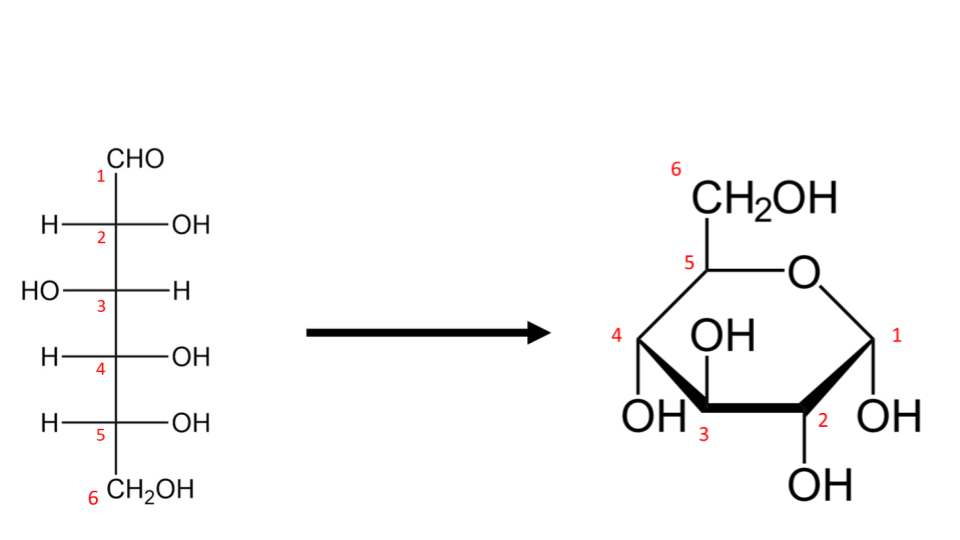
In reality however, these monosaccharide molecules do not exist as open- chains. The first and fifth carbon atoms in the chain fold over to form a hexagonal structure with the oxygen atom forming a bridge between these carbon atoms. This is the functional form of the D-glucose molecule.
Fig 5: The folding of an open-chain glucose molecule to a hexagonal glucopyranose unit
What’s in a Name?
Glycosidic Bonding
Now that we have a basic understanding of the structure of the monosaccharides, we can begin to understand how they link together to form polysaccharides. The bonds that link these monosaccharide molecules to one another are called glycosidic linkages or glycosidic bonds. These linkages are traditionally formed between the alcohol (-OH) containing first, third, fourth or sixth carbon atoms. So when a bond is called a 1 → 4 glycosidic bond, it indicates that the first and fourth carbon atoms of adjacent molecules are participating in the bond formation.
Alpha or Beta?
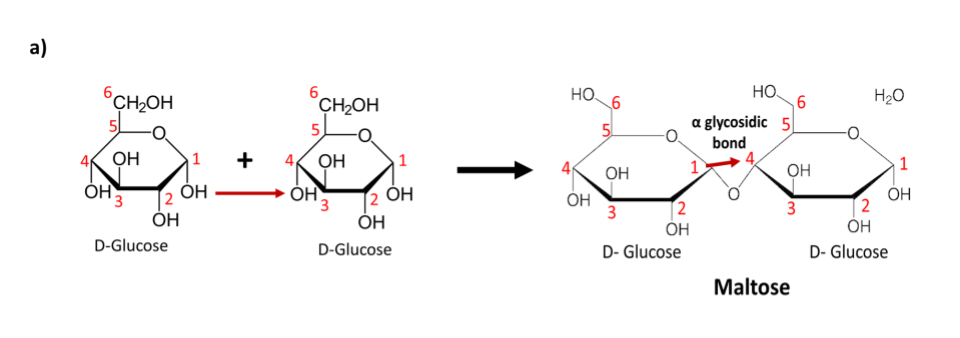
Another important aspect of the bond is its orientation. The roman letter alpha (𝛼) or beta (𝛽) are used to indicate this orientation. This can be better understood by considering the two disaccharides in the image below. Let us begin by imagining the ring to be a plane with the functional groups sticking out above and below this plane.
Figure 6 a) represents the link formed between adjacent D-glucose molecules in a Maltose molecule. The first and fourth carbon atoms of the adjacent glucose molecules partake in a 1→ 4 glycosidic bond. The (-OH) groups taking place in the bond lie on the same plane below the hexagonal ring structure. Bonds taking place in the same plane are termed 𝛼-glycosidic bonds. Therefore Maltose exhibits an 𝛼 (1→4) glycosidic bond between its two glucose monosaccharides.
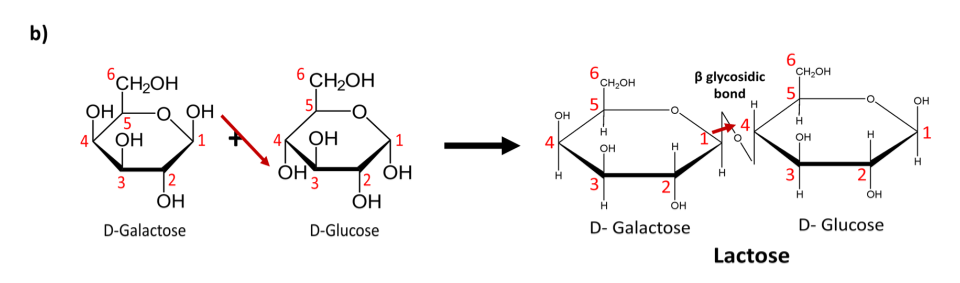
In contrast Lactose molecules (Fig 6 b)) are linked through 𝛽 (1→4) glycosidic bonds. The (-OH) molecules from galactose and glucose that take place in this bond formation lie on opposite sides of the hexagonal ring structure. This bond therefore forms across this plane.
Fig 6: a) The combination of two D-glucose molecules via an 𝛼 (1→4) glycosidic bond results in the formation of a Maltose molecule
b) The combination of D-galactose and D-glucose molecules via a 𝛽 (1→4) glycosidic bond results in the formation of a Lactose molecule
While this terminology can be rather confusing, the important thing to remember is that 𝛼 glycosidic bonds form on the same plane while 𝛽 glycosidic bonds form across two different planes. This difference contributes to very different three-dimensional conformations for the two kinds of bonds. 𝛼 bonds are more linear resulting in tightly packed structures as in the case of amylose (a component of cellulose), while 𝛽 bonds are more flexible and tend to rotate into helical shapes like in the case of chitin.
Different kinds of beta glucans
The above examples demonstrate the different kinds of bonds that can form between monosaccharide molecules. However, when we talk about bioactive polysaccharides from mushrooms, we are specifically interested in beta glucans.
Mushroom beta glucans are those polysaccharides that consist of glucose molecules linked together via 𝛽 (1→3) and 𝛽 (1→6) glycosidic bonds. This is an important distinguishing factor since beta glucans are also found in a wide variety of plant foods such as barley and oats.
These plant beta glucans consist primarily of 𝛽 (1→3) and 𝛽 (1→4) glycosidic linkages. The 𝛽 (1→4) linkages result in a more linear and packed structure. As a result, these plant glucans are harder to break down in our digestive tracts. They thus contribute to the beneficial insoluble fibers that nourish our microbiome.
Beta glucan diversity
Beta glucans are found in all fungi, including yeasts. Different fungi have different glucan compositions. Ascomycete fungi such as Cordyceps contain glucans that have varying amounts of glucose, galactose and mannose. Basidiomycete fungi such as oyster mushrooms, Reishi, Turkey tail and Shiitake have predominantly glucose in their glucan molecules with minor contributions from the other monosaccharides.
The ratios in which the different monosaccharides are found in the glucan molecules affect both their structures as well as their bioactivities. The different configurations and branching structures of beta glucans lead to differences in their effects on our health. These effects vary considerably from species to species. It is therefore not surprising that the species of mushrooms that have been used for medicinal purposes for the last two to three thousand years are also the ones which have the highest beta glucan content.
We always recommend our readers to look for beta glucan content in their mushroom extracts. Polysaccharide content does not imply beta glucans.
Common sources of beta glucans and their health benefits
If you’ve ever consumed a mushroom, you have consumed beta glucans. Many of the health benefits of consuming mushrooms come from the beta glucans. As beta glucans are an essential part of the fungal cell wall (see blog), every cell of the mushroom contains beta glucans. This makes them very easy to incorporate into our diets. All we have to do is consume mushrooms, although some ways of consumption lead to better absorption compared to others.
Oyster mushrooms
Oyster mushrooms are robust and easy to grow. They are a beginner friendly mushroom to try growing at home. They grow happily on almost any plant based substrate and are ready to burst out fruiting within a few weeks. (if you want to grow your own at home try our mushroom growing kits)
Fig 7: Pleurotus ostreatus (Pearl oyster mushroom) [image illustration]
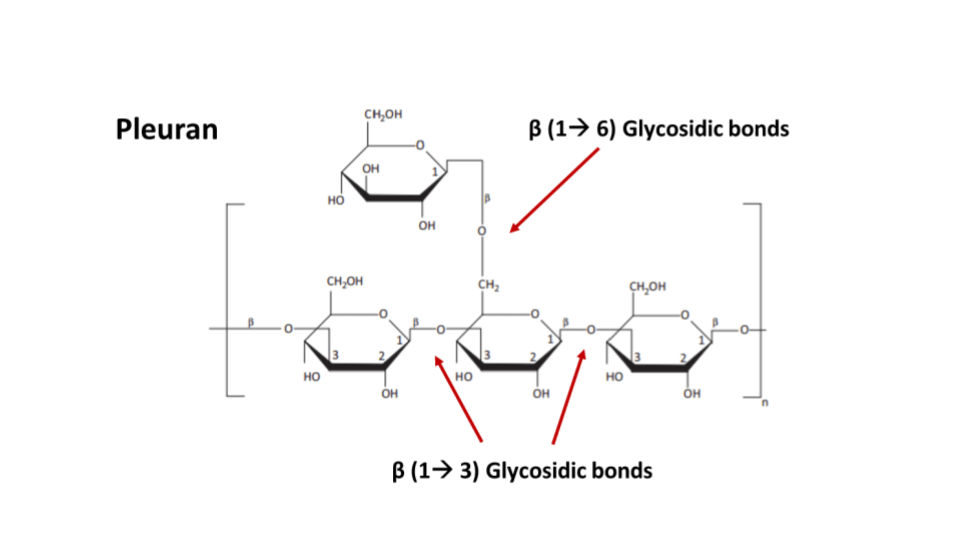
Pleuran is a beta glucan isolated from Pleurotus ostreatus (Pearl Oyster Mushroom). It has been shown to have an immunomodulatory effect while also being beneficial for skin, gut and respiratory health. It also has antiviral activity.
Fig 8: Molecular structure of Pleuran, a 𝛽 glucan extracted from oyster mushrooms (Source)
Shiitake Mushroom
Shiitake mushrooms are another edible variety that is rich in beta glucans. It has long been consumed by cultures of the far East and is revered both for its culinary and medicinal properties.
Fig 9: Lentinula edodes (Shiitake mushroom) [image illustration]
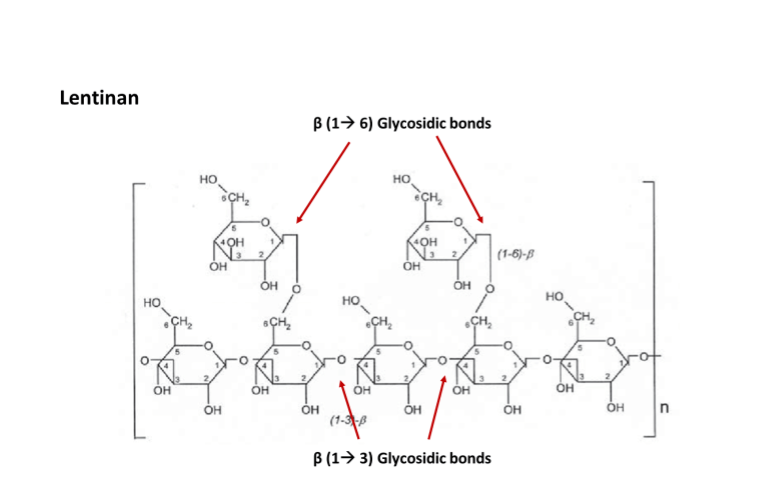
Lentinan is a beta glucan isolated from Lentinus edodes (Shiitake Mushroom). It has potent immunomodulatory effects. This in turn promotes the body’s own anti-cancer, antiviral and antimicrobial abilities. It is one of the most well understood beta glucans. Its widespread use is restricted by the difficulty of extraction of this molecule.
Fig 10: Molecular structure of Lentinan, a 𝛽 glucan extracted from shiitake mushrooms (source)
Maitake Mushroom
The Hen of the woods, also known as Maitake in Japan, is well known for its delicious taste and aroma. However it is also rich in beta glucans that provide immunomodulatory, antidiabetic. antiviral and antitumor properties.
Fig 11: Grifola frondosa (Maitake mushroom) [image illustration]
Grifolan is a beta glucan isolated from Grifola frondosa (Hen of the Woods mushroom). It has strong antitumor activity.
Reishi Mushroom
Apart from edible varieties, most medicinal mushrooms are particularly rich in beta glucans. The Reishi mushroom, also known as the herb of immortality in traditional Chinese medicine, has a very high beta glucan content. These beta glucans are in part responsible for the legendary immunomodulatory properties of Reishi mushroom extracts.
Fig 11: Ganoderma lucidum (Reishi mushroom) [image illustration]
Ganoderan is a beta glucan isolated from Ganoderma lucidum (Reishi mushroom). It possesses antitumor, hypoglycemic, antioxidant, probiotic and immunomodulatory properties.
Conclusion
Table 1: List of mushrooms with their beta glucans and associated health benefits
In this article we delved into the structural details of beta glucans. We took apart the differences between polysaccharides and beta glucans and looked at how the glucose molecule forms the fundamental building block for most beta glucan molecules of fungal origin. Finally, we explored some of the commonly studied beta glucans and the mushrooms they came from.
Beta glucans are extremely diverse in their structure. No two mushroom species will have the same beta glucan composition. These differences allow the extracts obtained from different mushroom species to have different health effects. In recent years many clinical trials have been initiated to investigate the relevance of beta glucan supplementation. Traditional healers swear by the healing powers of functional mushrooms. Modern medicine is attempting to verify and specify the use cases where these fungi can have maximum impact.
A major challenge lies in obtaining large quantities of pure beta glucan molecules. Only about 20% of the beta glucans are soluble without significant heating. Beta glucans consumed in the form of whole mushrooms are harder to digest. Extraction is a method of enriching beta glucans and increasing their bioavailability.
Many mushroom extracts represent their concentration as ratios or in terms of polysaccharide content. These are misleading terms and do not indicate the effectiveness of the extracts. We always recommend that one checks specifically for beta glucan content when inspecting an extract for potential health benefits. Always remember that polysaccharides do not imply beta glucans.
Modern extraction methods aim to maximize and standardize beta glucan content per dose. In our next blog we will explore the latest extraction technologies and how they can help improve beta glucan yields.
References
Š. Karácsonyi and Ľ. Kuniak. 1994. “Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble β-d-glucan”. DOI: 10.1016/0144-8617(94)90019-1
Milos Jesenak et. al. 2015. “β-Glucan-based cream (containing pleuran isolated from pleurotus ostreatus) in supportive treatment of mild-to-moderate atopic dermatitis”. DOI: 10.3109/09546634.2015.1117565
Ingrid Urbancikova et. al. 2020. “Efficacy of Pleuran (β-Glucan from Pleurotus ostreatus) in the Management of Herpes Simplex Virus Type 1 Infection”. DOI: 10.1155/2020/8562309
Juraj Majtan. 2013. “Pleuran (β- Glucan from Pleurotus ostreatus): An Effective Nutritional Supplement against Upper Respiratory Tract Infections?”. DOI” 10.1159/000341967
Yangyang Zhang et. al. 2011. “Advances in lentinan: Isolation, structure, chain conformation and bioactivities”. DOI: 10.1016/j.foodhyd.2010.02.001
Takuma Sasaki and Nobuo Takasuka. “Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes”. DOI: 10.1016/S0008-6215(00)83552-1
Yiran Zhang et. al. 2018. “Lentinan as an immunotherapeutic for treating lung cancer: a review of 12 years clinical studies in China”. DOI: 10.1007/s00432-018-2718-1
Hiroshi Hikino et. al. 1989. “Mechanisms of Hypoglycemic Activity of Ganoderan B: A Glycan of Ganoderma lucidum Fruit Bodies”. DOI: 10.1055/s-2006-962057
Hiroshi Hikino et. al. 1985. “Isolation and Hypoglycemic Activity of Ganoderans A and B, Glycans of Ganoderma lucidum Fruit Bodies”. DOI: 10.1055/s-2007-969507
Masashi Tomoda et. al. 1986. “Glycan structures of ganoderans b and c, hypoglycemic glycans of ganoderma lucidum fruit bodies”. DOI: 10.1016/S0031-9422(00)83748-6
Xue-Song Yang et. al. 2009. “Effects of Ganoderan on the dysbacteria in intestinal tract of mice”. DOI: 10.1096/fasebj.23.1_supplement.980.2
Weifeng Wang et. al. 2019. “Ganoderan (GDN) Regulates The Growth, Motility And Apoptosis Of Non-Small Cell Lung Cancer Cells Through ERK Signaling Pathway In Vitro And In Vivo”. DOI: 10.2147/OTT.S221161
Yifeng Zhang et. al. 2013. “Schizophyllan: A review on its structure, properties, bioactivities and recent developments”. DOI: 10.1016/j.bcdf.2013.01.002





 Neeladri Chowdhury
Neeladri Chowdhury


